June Bulletin
Issue 105
Community Notices
Thank you for attending the 2025 Marble Center poster symposium!
On May 20, 2025, the MIT community participated in a poster symposium organized by the Marble Center for Cancer Nanomedicine to discuss nano- and precision-based approaches for the early detection and treatment of a variety of diseases, including cancer. 14 research posters were selected for this event, representing 18 research investigators from various institutes and departments at MIT, the Koch Institute, the Broad Institute, Beth Israel Deaconess Medical Center, Scripps Research Institute, Dana–Farber/Harvard Cancer Center, the Ragon Institute, and Brigham and Women’s Hospital, among many others.
We are grateful for our wonderful panel of judges —Drs. Parthiban Rajasekaran (Sanofi), Liangliang Hao (Boston University), Megan Vierhout (Novartis Biomedical Research), and Allen Jiang (Broad Institute)—and the amazing group of scientists who presented at the event!


















Dr. Angela Belcher’s speaks at the 2025 MIT Health Science Forum
Dr. Angela Belcher leads research focused on understanding and harnessing nature’s own processes in order to design technologically important materials and devices for energy, the environment, and medicine.
Reminder: Register for the 2025 Koch Institute Symposium
The 23rd Annual Koch Institute Symposium on Antibody Drug Conjugates: Targeted Cancer Therapies at the Intersection of Chemistry, Biology, and Engineering will take place on Friday, June 27, 2025 (Huntington Hall, 10-250). Kicking off with 2022 Nobel Prize winner Carolyn Bertozzi, our speaker lineup features an array of industry and academic leaders who will discuss current advancements and challenges in deploying antibody drug conjugates (ADCs) as anti-cancer agents. We cordially invite scientists, oncologists, and any other member of the biomedical community to join us in a day of engaging talks, stimulating conversations with colleagues, and of course camaraderie in our collective fight against cancer.
News
The National Academies publish the quadrennial review of the U.S. National Nanotechnology Initiative
On May 20, 2025, the National Academies of Sciences, Engineering, and Medicine (NASEM) announced the release of a report entitled Quadrennial Review of the National Nanotechnology Initiative (2025): Securing U.S. Global Leadership Through Renewed and Expanded Infrastructure. Requested by Congress as part of the 21st Century Nanotechnology Research and Development Act, the report focuses on the infrastructure of the National Nanotechnology Initiative (NNI). The Committee on the Quadrennial Review of the NNI “recommends a new focus on renewing and expanding the nanotechnology infrastructure, including instruments, facilities, and people, so that the intellectual capital of nanotechnology can be converted into economic, social, and national security gains for the United States.” According to the report, this conclusion “reflects a consideration of the suitability of the nation’s existing nanotechnology infrastructure for current and emerging needs in academia and industry” and rests on the Committee’s “analysis of the existing nanotechnology infrastructure users in academia and beyond[,] as well as the existing barriers that limit the impact and accessibility of the infrastructure. Read more…
Particles carrying multiple vaccine doses could reduce the need for follow-up shots
Representative high-resolution light microscopy images showing an array of empty bases pressed from polyanhydride polymer film and sealed microparticles. Credits: Image: Courtesy of the researchers
(Anne Trafton | MIT News) Around the world, 20 percent of children are not fully immunized, leading to 1.5 million child deaths each year from diseases that are preventable by vaccination. About half of those underimmunized children received at least one vaccine dose but did not complete the vaccination series, while the rest received no vaccines at all. To make it easier for children to receive all of their vaccines, MIT researchers are working to develop microparticles that can release their payload weeks or months after being injected. This could lead to vaccines that can be given just once, with several doses that would be released at different time points.
In a study appearing in the journal Advanced Materials, the researchers showed that they could use these particles to deliver two doses of diphtheria vaccine — one released immediately, and the second two weeks later. Mice that received this vaccine generated as many antibodies as mice that received two separate doses two weeks apart. The researchers now hope to extend those intervals, which could make the particles useful for delivering childhood vaccines that are given as several doses over a few months, such as the polio vaccine. “The long-term goal of this work is to develop vaccines that make immunization more accessible — especially for children living in areas where it’s difficult to reach health care facilities. This includes rural regions of the United States as well as parts of the developing world where infrastructure and medical clinics are limited,” says Ana Jaklenec, a principal investigator at MIT’s Koch Institute for Integrative Cancer Research. Read more…
Research highlights in the nanomedicine world
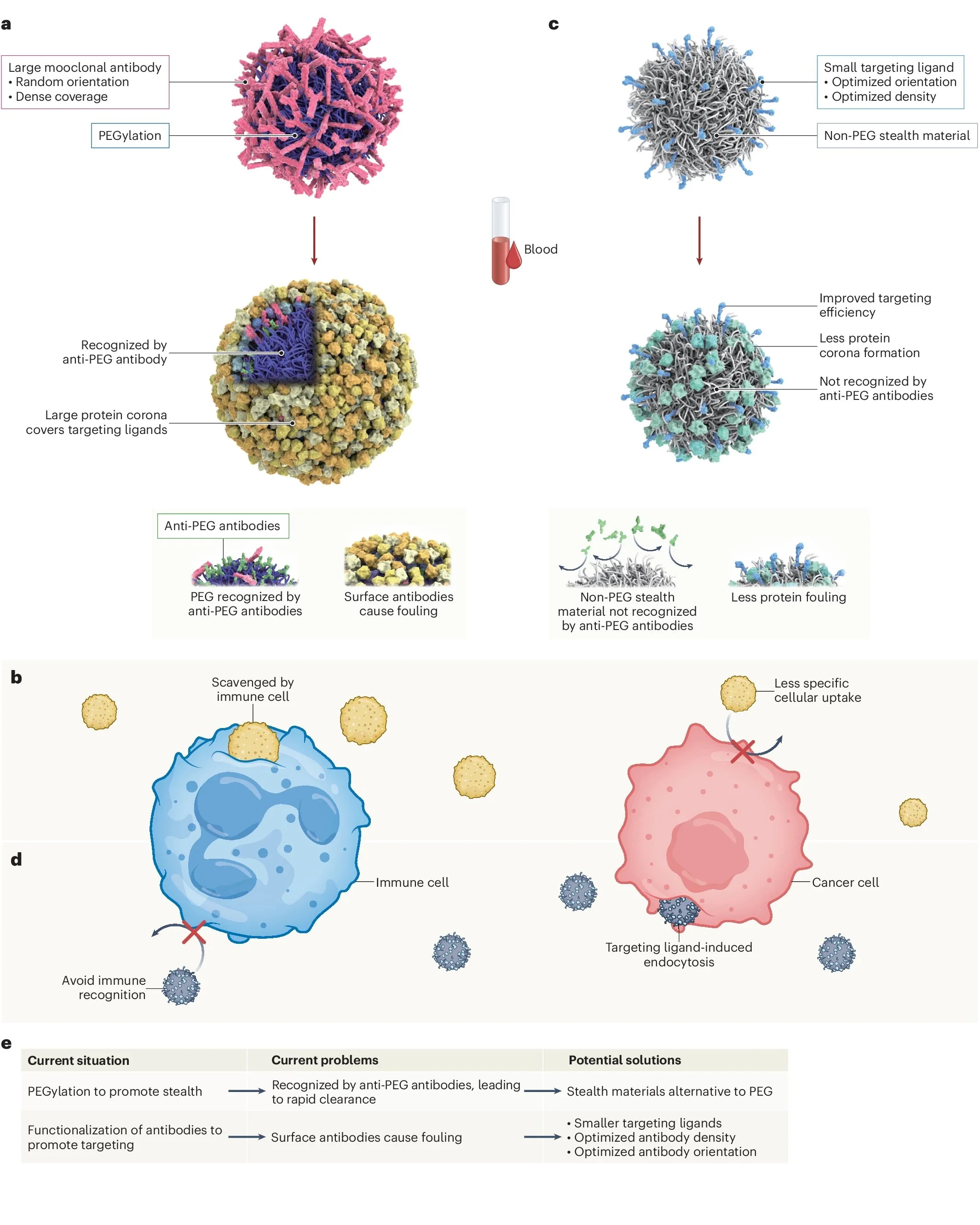
(Editorial, Nature Nanotechnology 20, 575 (2025)) The benefits and risks of PEGylation in nanomedicine: Polyethylene glycol (PEG) is a commonly used coating agent in nanomedicine, but there are growing concerns about its immunogenicity. Two Comment articles discuss the issue and possible alternatives to PEG:
In their Comment, Ju and Kent examine the balance between PEG-induced stealth properties and targeting efficiency. PEG coating reduces nonspecific protein binding and accelerated blood clearance, enhancing the stability of nanoparticles in the bloodstream. At the same time, functionalizing PEGylated nanoparticles with antibodies increases targeting efficiently, but it can compromise stealth. An optimal balance may be achieved by fine-tuning the PEG:antibody ratio, PEG chain length and molecular weight, ligand orientation, and ligand size. Alternatively, engineering an ad hoc artificial protein corona or a better understanding of naturally formed ones could drive organ-specific targeting.
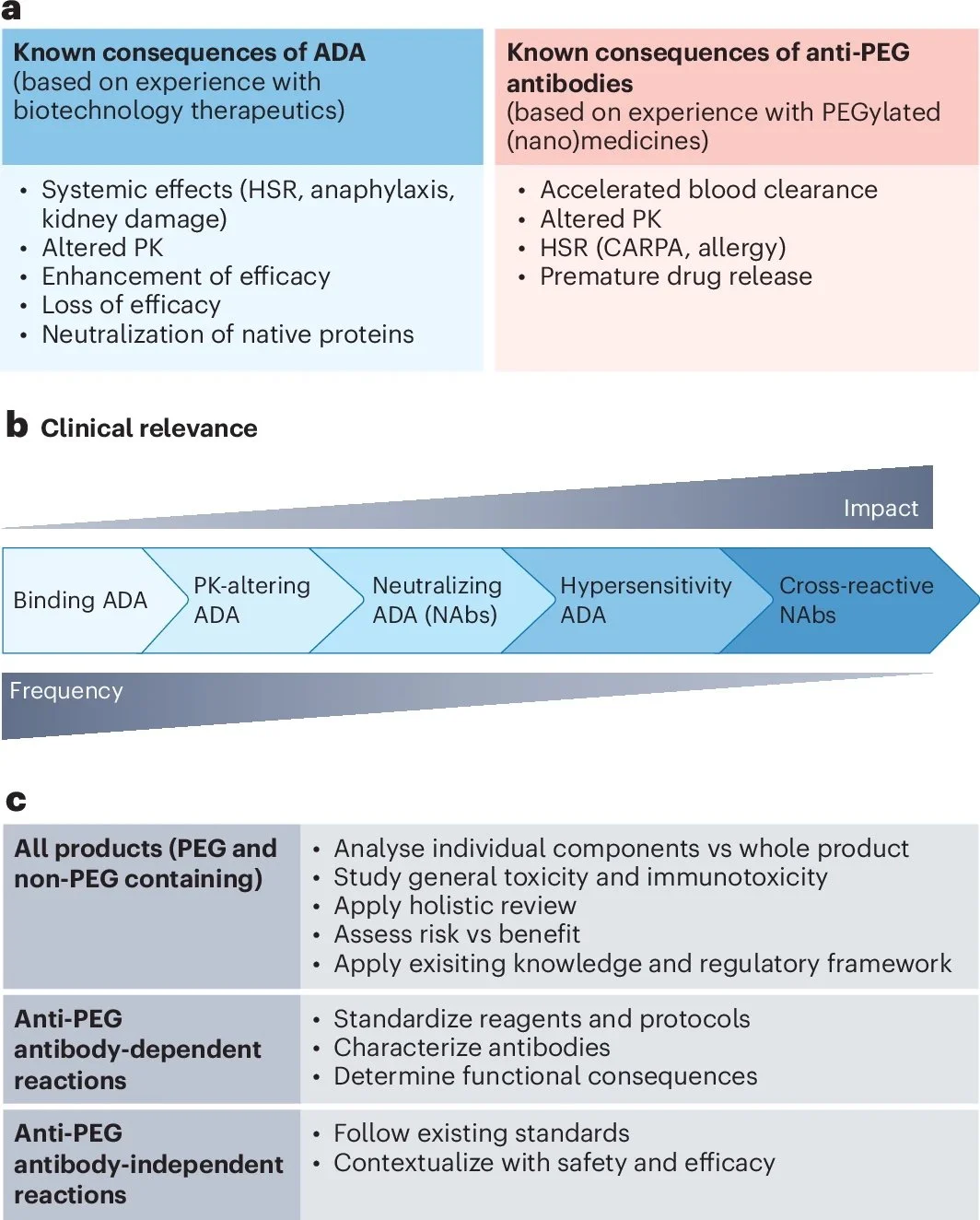
In a second Comment, Dobrovolskaia also discusses the issue of potential immunogenicity of the PEG alternatives and raises additional points. There are two major gaps in knowledge that need to be addressed before considering alternatives. One is the lack of a standardized methodology, control, and reagents for the detection of binding antibodies. The term ‘immunogenicity’ is often misused in the literature to refer to cytokine production, which, while easier to measure, does not necessarily correlate with antigen-specific cellular or humoral response. There is therefore the need to clearly evaluate whether a product is immunogenic (that is, generates antibodies) or immunostimulant (that is, leads to cytokine release without antibody production). The second gap is the need for a deeper understanding of the mechanisms leading to PEG immunogenicity. This includes analysing the nature of the generated antibodies, as not all antibodies are functionally equivalent, with different subclasses and isotypes displaying various degrees of immunotoxicity. Additionally, whether PEG is free, or conjugated to a protein or to a nanoparticle can affect antibody generation.
Job opportunities
Scientist, Portal Bio. Portal is a start-up cell engineering platform company focused on enabling next generation cell engineering and cell analytics across research and clinical applications. Our initial product suite is based on a proprietary mechanical delivery system capable of delivering many different types of cargo to a broad range of cell types.. As we grow our team, we are seeking an innovative and motivated scientist with a molecular biology and cell engineering background to join our group in developing a library of molecules that would enable transient cell modifications complementary to editing technologies. This scientist will lead design and validation of modification strategies, including mRNA and siRNA, that could enable long term changes in cell function or performance through only transient engineering. This position will also support future clinical implementation with clinical and corporate partners. The successful candidate has a passion for early-stage research and thrives in a fast-paced, collaborative environment.
Funding opportunities
| Funding Source | Grant ID | Deadline | MIT HEALs Graduate Fellowship | N/A | June 10, 2025 | 2025 Annual Marble Center RFP | N/A | July 21, 2025 |
|---|